AutoDesk® Pure Chromatography System
Autodesk®Pure tabletop automatic chromatography system is a product specially designed for purification and preparation of laboratory level biological samples
AutoPrep automatic chromatography system is a pilot and large-scale purification system for MAB, insulin, blood products,vaccines,recombinant proteins and other biological products. As a complete set of integrated control unit, it can realize the automatic balance, sample loading, elution, collection, regeneration, cleaning and other purification processes of column , and comply with
the current GMP laws and regulations. The system is available in a variety of flexible configurations to accommodate large-scale production needs.
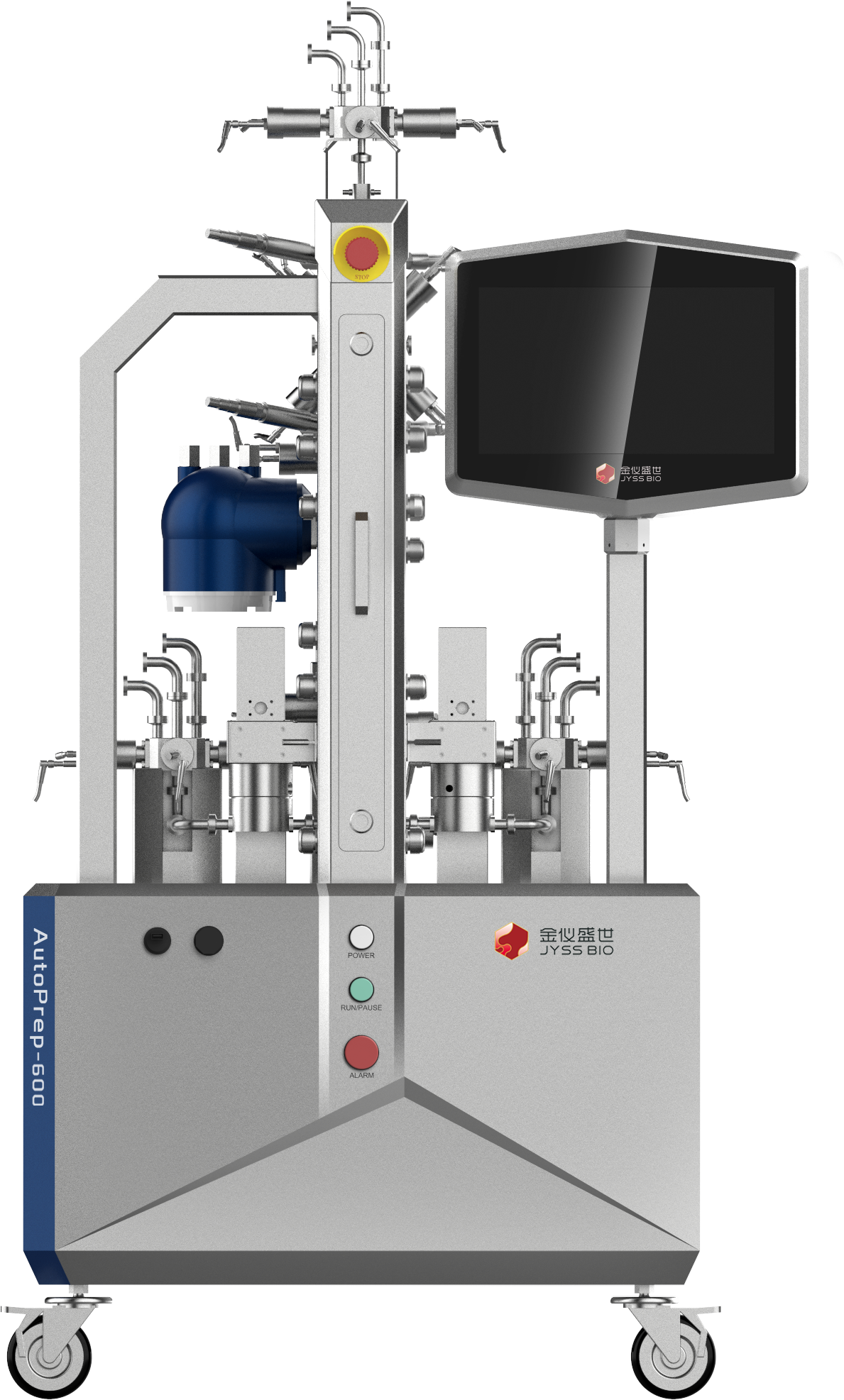
Features and Advantages

Autodesk®Pure tabletop automatic chromatography system is a product specially designed for purification and preparation of laboratory level biological samples
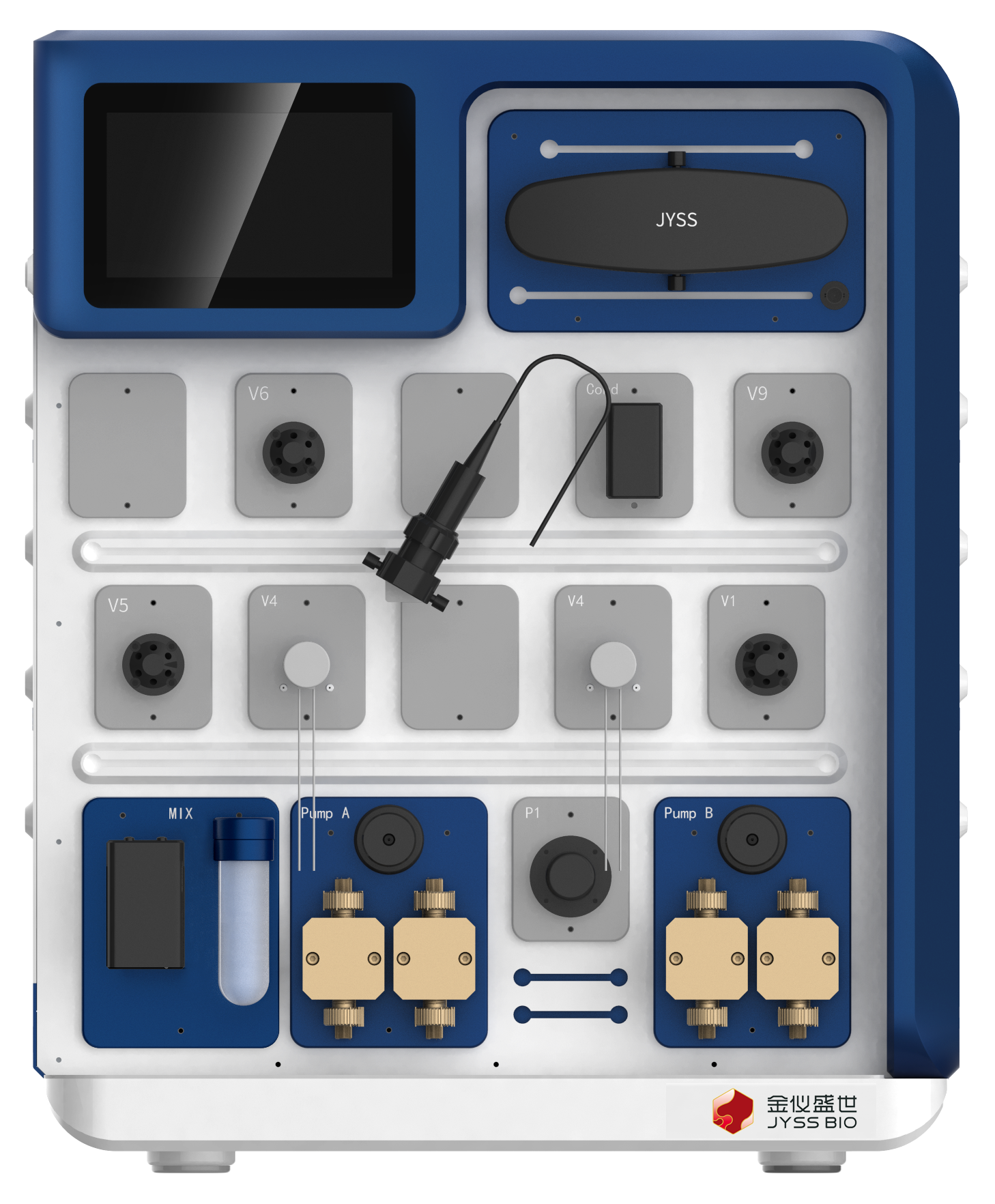
Autodesk®Prime lab automatic chromatography system is a cost-effective chromatography system